Which Of The Following Distinguishes Hydrogen Bonds From Covalent Bonds . Water acts as a solvent because the partial negative charge on the oxygen in water attracts sodium, while the partial positive charge on hydrogen attracts chloride. A single covalent bond between two atoms is due to an electron pair shared by the atoms.
Difference Between Covalent And Hydrogen Bonds | Definition, Formation Of Bond, Examples from pediaa.com
Which of the following correctly ranks the types of chemical bonds, in order, from strongest to weakest? Only ionic bonds can form within molecules.
Difference Between Covalent And Hydrogen Bonds | Definition, Formation Of Bond, Examples
Suspensions may also be called emulsions. Hydrogen bonds are formed between molecules when hydrogen is covalently bonded to an element that has a In this case the electronegativity difference is low.
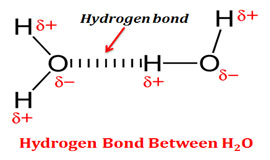
Source: slideplayer.com
Therefore, it will form covalent bonds with three hydrogen atoms. Two hydrogen atoms join together to form a molecule of hydrogen gas. The two strands of the double helix would separate b.
Source: pediaa.com
• hydrogen atom should be there to have a hydrogen bond. Which of the following distinguishes hydrogen bonds from covalent bonds? Which factor distinguishes a metallic bond from an ionic bond or a covalent bond how do electronegativity values determine the charge distribution in a polar covalent bond the sun produces energy by fusing hydrogen atoms into ____ atoms in.
Source: www.quora.com
Involves the sharing of electron(s) between elements. The three electrons contributed by the hydrogen atoms will fill the valence shell of nitrogen. Hydrogen bonds are betweem molecules and are weak forces.
Source: www.sciencedirect.com
Only hydrogen bonds can form between molecules. Ionic bonds hold together compounds that do not dissolve in water, whereas covalent bonds hold together molecules that dissolve in water. D) only hydrogen bonds can.
Source: onlychemistrydiscussion.blogspot.com
Which of the following is not a result of hydrogen bonds? Which of the following correctly ranks the types of chemical bonds, in order, from strongest to weakest? Metallic bonds are the chemical bonds that join metals to metals.
Source: pediaa.com
Which of the following distinguishes hydrogen bonds from covalent bonds? Recall the definition of a hydrogen bond and covalent bond. The purines would be separated from the deoxyribose sugar d.
Source: www.easybiologyclass.com
B) only covalent bonds can form between molecules. Involves the sharing of electron(s) between elements. Two hydrogen atoms join together to.
Source: www.coursehero.com
• covalent bonds result between atoms to produce a molecule. Hydrogen bonds can be seen between molecules. The covalent bond is also called a shared bond.
Source: www.chemistryworld.com
Two hydrogen atoms join together to form a molecule of hydrogen gas. The covalent bond is also called a shared bond. What would first happen to dna molecules treated with dnaase?
Source: socratic.org
Which of the following distinguishes hydrogen bonds from covalent bonds? Dnaase is an enzyme that catalyzes the hydrolysis of the covalent bonds that join nucleotides together. Only hydrogen bonds can form within molecules.
Source: www.chemistrylearner.com
The ionic bond is the electrostatic force of attraction between two oppositely charged ions. A single covalent bond between two atoms is due to an electron pair shared by the atoms. Which factor distinguishes a metallic bond from an ionic bond or a covalent bond how do electronegativity values determine the charge distribution in a polar covalent bond the sun.
Source: www.britannica.com
Each single covalent bond includes the sharing of three pairs of electrons. Only hydrogen bonds can form within molecules. Traditionally one distinguishes the following types of bonds:
Source: courses.lumenlearning.com
The covalent bonds are intramolecular bonds because they hold the atoms together in a single molecule. They occur between, for instance, hydrogen, carbon, nitrogen, and oxygen. Individual hydrogen bonds are weak bonds however, their presence in large number provide them considerable strength.
Source: www.youtube.com
What distinguishes hydrogen bonds from covalent bonds? • covalent bonds are stronger than hydrogen bonds. Each single covalent bond includes the sharing of three pairs of electrons.
Source: pediaa.com
A) only ionic bonds can form within molecules. Only hydrogen bonds can form between molecules. Ionic bonds hold together compounds that do not dissolve in water, whereas covalent bonds hold together molecules that dissolve in water.
Source: www.coursehero.com
Ionic bonds hold together compounds that do not dissolve in water, whereas covalent bonds hold together molecules that dissolve in water. Hydrogen bonds are not exactly bonds, but they can be better considered as forces of attraction. Covalent bonds form when two atoms share electrons.
Source: www.easybiologyclass.com
Nitrogen has 5 electrons in its valence (outermost) electron shell. Covalent, ionic, hydrogen which of the following distinguishes hydrogen bonds from covalent bonds? B) only covalent bonds can form between molecules.
Source: www.sciencedirect.com
Only hydrogen bonds can form within molecules. Covalent, ionic, hydrogen which of the following distinguishes hydrogen bonds from covalent bonds? A)ionic bonds form between the same two elements, whereas covalent bonds form between different elements.
Source: www.coursehero.com
Suspensions may also be called emulsions. Covalent bonds are stronger than hydrogen bonds. C.) only hydrogen bonds can form between molecules.
Source: vivadifferences.com
Hydrogen bonds are especially strong intermolecular forces. Metallic bonds are the chemical bonds that join metals to metals. Covalent bonds can be occurred between any two atoms.