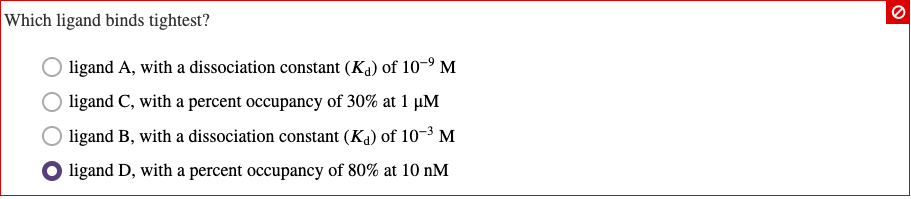
Which Ligand Binds Tightest . To answer this item, we must take note that the ligand that binds the tightest is the one with the lowest dissociation constant, kd. It helps to know which ligand binds the tightest.
Solved Which Ligand Binds Tightest? Ligand D, With A Percent | Chegg.com from www.chegg.com
Ligand d, with a percent occupancy of 80% at 10 nm. One bacterial protein called streptavidin binds to the biotin ligand extremely tightly.
Solved Which Ligand Binds Tightest? Ligand D, With A Percent | Chegg.com
Ligand binding changes amplitude, position and width of reflected lights. Ligand d, with a percent occupancy of 80% at 10 nm. Which ligand binds the tightest?
Source: naijaeduinfo.com
Ligand a, with a dissociation constant ( kd ) of 10−9 m ligand d, with a percent occupancy of 80% at 10 nm ligand b, with a dissociation constant ( kd ) of 10−3 m ligand c, with a percent occupancy of 30% at 1 μm C) ligand c, with a percent occupancy of 30% at 1 um. The ic50.
Source: www.researchgate.net
One bacterial protein called streptavidin binds to the biotin ligand extremely tightly. 0.3 = (1x10⁻⁶)/ (1x10⁻⁶ + kd) ; Ligand c, with a percent occupancy of 30% at 1 μm
Source: www.chegg.com
(d) ligand d, with a per Ligand c, with a percent occupancy of 30% at 1 μm Ligand binding changes amplitude, position and width of reflected lights.
Source: www.slideserve.com
In other words, the value represents the amount of inhibitor needed to. In some cases, the associations can be quite strong—for example, the. One bacterial protein called streptavidin binds to the biotin ligand extremely tightly.
Source: www.numerade.com
One bacterial protein called streptavidin binds to the biotin ligand extremely tightly. One bacterial protein called streptavidin binds to the biotin ligand extremely tightly. Nm is (10'm), um is (10°m) and mm is (103m) a.
Source: www.nature.com
When the immobilized ligand binds an analyte in solution, this local refractive index increases in direct proportion to the number of molecules bound to the sensor, making the refractive index shift equivalent to a change in mass. (c) ligand c, with a percent occupancy of 30% at one micromolar. Therefore we can conclude that protein a binds ligand tighter at.
Source: study.com
The ic50 value represents the concentration at which 50% of maximum possible inhibition occurs for a process. Nm is (10'm), um is (10°m) and mm is (103m) a. D) ligand d, with a percent occupancy of 80% at 10 um.
Source: chemistry-europe.onlinelibrary.wiley.com
0.3 = (1x10⁻⁶)/ (1x10⁻⁶ + kd) ; Ligand b, with a dissociation constant ( 𝐾d ) of 10−3 m d. Nm is (10'm), um is (10°m) and mm is (103m) a.
Source: bio.libretexts.org
Kd= [p] [l]/ [pl] (p=protein, l=ligand) fraction of bonding = [l] / [l] + kd. Ligand c, with a percent occupancy of 30% at 1 μm Therefore we can conclude that protein a binds ligand tighter at the midpoint of the binding curve.
Source: www.researchgate.net
We'd like to design proteins that can bind ligands just as tightly, but this is a difficult protein design challenge. C) ligand c, with a percent occupancy of 30% at 1 um. Ligand a, with a dissociation constant (𝐾dkd) of 10−9 m10−9 m.
Source: www.numerade.com
0.3 = (1x10⁻⁶)/ (1x10⁻⁶ + kd) ; Ligand a, with a dissociation constant ( 𝐾d ) of 10−9 m b. Pwr has lower sensitivity than spr with regard to refractive index, thickness and mass parameters.
Source: quizlet.com
When the immobilized ligand binds an analyte in solution, this local refractive index increases in direct proportion to the number of molecules bound to the sensor, making the refractive index shift equivalent to a change in mass. In addition, the limited dynamic range of visual readouts of gels that are often used to evaluate. One bacterial protein called streptavidin binds.
Source: quizlet.com
This effect is of positive cooperativity. Ligand d, with a percent occupancy of 80% at 10 nm. The method described herein not only picks out the tightest binding ligands, it can also be used to detect ligands with.
Source: www.chegg.com
Kd's for both a and b are already given so, we only need to solve kds for c and d. Ligand c, with a percent occupancy of 30% at 1 μm We'd like to design proteins that can bind ligands just as tightly, but this is a difficult protein design challenge.
Source: www.chegg.com
To answer this item, we must take note that the ligand that binds the tightest is the one with the lowest dissociation constant, kd. It is formed when atoms or molecules bind together by sharing of electrons. In some cases, the associations can be quite strong—for example, the.
Source: www.chegg.com
If you add the central ion to a solution of the tighter binding ligand first, some of the ions will be occupied by multiple strong ligands, since the relative concentrations of the two will favor the ligand at first. Ligand d, with a percent occupancy of 80% at 10 nm In some cases, the associations can be quite strong—for example,.
Source: quizlet.com
When the immobilized ligand binds an analyte in solution, this local refractive index increases in direct proportion to the number of molecules bound to the sensor, making the refractive index shift equivalent to a change in mass. One bacterial protein called streptavidin binds to the biotin ligand extremely tightly. Ligand binding changes amplitude, position and width of reflected lights.
Source: www.chegg.com
We'd like to design proteins that can bind ligands just as tightly, but this is a difficult protein design challenge. The ic50 value represents the concentration at which 50% of maximum possible inhibition occurs for a process. C) ligand c, with a percent occupancy of 30% at 1 um.
Source: www.chegg.com
(d) ligand d, with a per Therefore we can conclude that protein a binds ligand tighter at the midpoint of the binding curve. The method described herein not only picks out the tightest binding ligands, it can also be used to detect ligands with.
Source: www.chegg.com
Ligand b, with a dissociation constant ( 𝐾d ) of 10−3 m d. It is formed when atoms or molecules bind together by sharing of electrons. Therefore we can conclude that protein a binds ligand tighter at the midpoint of the binding curve.